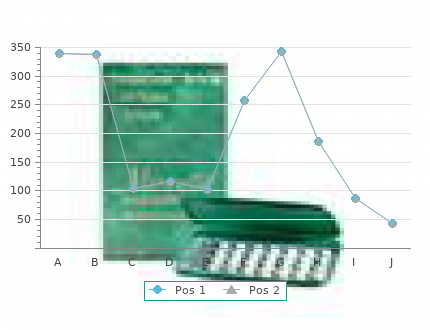

ECOSHELTA has long been part of the sustainable building revolution and makes high quality architect designed, environmentally minimal impact, prefabricated, modular buildings, using latest technologies. Our state of the art building system has been used for cabins, houses, studios, eco-tourism accommodation and villages. We make beautiful spaces, the applications are endless, the potential exciting.
By A. Mezir. Westwood College ó Illinois. 2018.
Mater- with a live birth rate of over 70% buy plendil 10 mg with amex pulse pressure refers to, and low-dose nal age and fetal loss: population based register linkage heparin may be beneficial for women with recur- study buy cheap plendil 2.5 mg online blood pressure medication for young adults. Risk rent miscarriage associated with factor V Leiden and factors for spontaneous abortion and its recurrence. Am APCR but is not readily available in many low-re- J Epidemiol 1988;128:420‚Äď30 source settings. A longitudinal fer benefits for women with one or more study of pregnancy outcome following idiopathic recur- mid-trimester losses as a result of cervical incompet- rent miscarriage. Cervical length ultrasound screening during women with recurrent spontaneous abortions and pregnancy may be useful in equivocal cases, but in- women with favorable reproductive histories. Am J sertion of cerclage in low-risk women with a short Public Health 1986;76:986‚Äď91 cervix on ultrasound alone (without a history of 13. Pregnancy: an mid-trimester pregnancy loss) is not recommended. Hum Reprod 1994;9:1328‚Äď32 no underlying systematic causes for the recurrent 14. Couples should be reassured that rent fetal aneuploidy and recurrent miscarriage. Obstet the chance of a successful pregnancy outcome after Gynecol 2004;104:784 three consecutive miscarriages is good, and support- 15. Maternal age-specific rates of numerical chromosome abnormalities with special ive care in couples with unexplained miscarriage is reference to trisomy. Human Genet 1985;70:11‚Äď17 associated with an excellent prognosis. Paternal age and treatments should be avoided, not only because maternal age are risk factors for miscarriage: results of they are not proven to be effective and usually incur a multicentre European study. Hum Reprod 2002;17: a cost, but also because adverse outcomes have been 1649‚Äď56 17. Future pregnancy out- reported (as with diethylstilbestrol many years ago! Hum Reprod 1997;12:387‚Äď9 locally, onward referral to a specialist center (if 18. Effects of ex- available) may confer benefits in selected couples posure to environmental tobacco smoke on reproduc- when resources allow. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol REFERENCES Scand 2003;82:182‚Äď8 1. Moderate Development (Monographs in Epidemiology and Biostatistics, alcohol intake during pregnancy and the risk of stillbirth vol. Oxford: Oxford University Press, 1989 and death in the first year of life. Cytogenetic 2002;155:305‚Äď12 analysis of miscarriages from couples with recurrent 21. A review of the literature relating miscarriage: a case‚Äďcontrol study. Hum Reprod 2002;17: caffeine consumption by women to their risk of repro- 446‚Äď51 ductive hazards. Obesity is associated under ultrasound guidance to improve the cytogenetic with increased risk of first trimester and recurrent study of early pregnancy failure. Hum Reprod 2002;17: miscarriage: matched case‚Äďcontrol study. Obesity: impact on clinical use of karyotyping spontaneous abortions. Obstetrician Gynaecologist Obstet Gynecol 2003;189:397‚Äď400 2009;11:25‚Äď31 144 Recurrent Miscarriage including Cervical Incompetence 24. Body carrier of a structural chromosome rearrangement.
A significant difference in median time to relapse was not found between groups continuing with risperidone augmentation and those who returned to citalopram monotherapy (102 days compared with 85 days; P=0 discount 10 mg plendil amex hypertension orthostatic. However buy plendil 10 mg without prescription heart attack names, findings from post-hoc subgroup analyses performed on data from the risperidone trial indicated that level of resistance to antidepressant treatment may have been a mitigating factor. In the subgroup of participants who were ‚Äúfully nonresponsive‚ÄĚ (less than 25% reduction in HAM-D-17), time to relapse was significantly greater for risperidone augmentation (97 days) than placebo (56 days, P=0. Atypical antipsychotic drugs Page 105 of 230 Final Report Update 3 Drug Effectiveness Review Project Suicide and suicidal ideation Compared with placebo, no statistically significant advantage in reducing suicidal ideation or suicide was found for aripiprazole, risperidone, or extended-release quetiapine. Suicides and 456 suicidal ideation outcomes were found for aripiprazole in a poster that reported a pooled analysis based on data from two 6-week, placebo-controlled trials of adjunctive treatment in 433, 439 adults with a history of inadequate response to antidepressant medication. In the pooled 456 analysis of adjunctive aripiprazole (N=737) compared with placebo, there were no suicides in either group, nor did any patient demonstrate treatment-emergent suicidal ideation based on the criterion of a score of 5 or greater on item 10 of the MADRS (score of 6, ‚ÄúExplicit plans for suicide when there is an opportunity‚ÄĚ). Incidence rates of treatment-emergent suicidal ideation were somewhat lower for aripiprazole (3. Rates of treatment-emergent, suicide-related, adverse events were 0% and 0. Both suicide-related adverse events in the placebo group were reported as suicidal ideation. There was also no significant difference between maintenance treatment with extended-release quetiapine or placebo monotherapy in suicidal ideation (data not 450 reported) based on findings from an unpublished trial. The effect of adjunctive risperidone on suicidal ideation was also evaluated in a small trial of 23 adults with severe depression (MADRS mean score of 35. In this trial, there was a trend toward risperidone augmentation superior to placebo (P=0. Functional capacity Functional capacity outcomes were found for aripiprazole, olanzapine, risperidone, and extended-release quetiapine. In all trials, functional capacity was measured based on the Sheehan Disability Scale (SDS). In the longest-term trial (unpublished, N=776), with up to 52 weeks of follow-up, maintenance treatment with extended-release quetiapine monotherapy was superior to 450 placebo in maintaining improvement in the SDS Total Score (data not reported). In adults with inadequate response to antidepressants, shorter-term evidence was found in 432, 433, 439 3 trials of aripiprazole given in combination with various antidepressants, 2 trials of 446 olanzapine given in combination with fluoxetine (in 1 publication), and in 1 trial of 438 risperidone given in combination with various antidepressants. The Family subscale was the only domain for which a statistically significant improvement was found compared with placebo across all trials of the 3 different atypical antipsychotics. Conversely, for the Work/School domain, no statistically significant improvements were found in any of the trials. On the Total Score, compared with placebo, improvements were significantly greater for adjunctive 439 438 aripiprazole in 1 of 3 trials and for adjunctive risperidone. Compared with placebo, 432, 439 significant improvements on the Social subscale were found in 2 of 3 trials of aripiprazole 438 and in the trial of risperidone. Findings on the Social subscale were not reported for the trials of olanzapine given in combination with fluoxetine, rather a significantly greater improvement 446 on the ‚Äúleisure item‚ÄĚ was described. Atypical antipsychotic drugs Page 106 of 230 Final Report Update 3 Drug Effectiveness Review Project Quality of life Compared with placebo, significant improvements in quality-of-life outcomes were found in 2 of 446 2 trials of olanzapine given in combination with fluoxetine (reported in 1 publication) and in 1 438 of 1 trial of risperidone given in combination with various antidepressants, whereas for 451 448, 449, 451, extended-release quetiapine, significant improvement was only found in 1 of 5 trials 454 430, 431 when given as monotherapy and neither of 2 trials when given in combination with ongoing antidepressant therapy. Based on pooled data from the SF-36 in adults with a history of inadequate response to antidepressants, 8-week improvements were significantly greater for combination therapy with olanzapine and fluoxetine compared with fluoxetine monotherapy on the Physical Summary Score (P=0. On the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q), 6-week Total Score improvements were significantly greater for adjunctive risperidone compared with placebo (mean difference, 5. For extended-release quetiapine, statistical superiority over placebo for improvement in quality of life was only established in 1 unpublished trial, when it was given as monotherapy in 451 older adults with a mean age of 71. Least squares means change on the Q-LES-Q Total Scores were significantly greater for extended-release quetiapine (+16. Response rates were reported in 436, 442, 447, 452 all but 4 of the acute treatment trials. The majority of trials defined response as a 50% or greater reduction in the MADRS.
Most other trials do not indicate a faster onset of action of one second-generation antidepressant compared with another plendil 10mg online pulse pressure 85. Dysthymia Comparative efficacy Poor No head-to head evidence exists purchase 10mg plendil fast delivery pulse pressure too low. Findings from five placebo-controlled trials were insufficient to draw conclusions about comparative efficacy. Comparative effectiveness Poor One fair effectiveness study provides mixed evidence about paroxetine vs. Subsyndromal depression Comparative efficacy Poor One nonrandomized, open-label trial did not detect any difference between citalopram and sertraline. Findings from two placebo-controlled trials were insufficient to draw conclusions. Comparative effectiveness No evidence Seasonal affective disorder Comparative efficacy Poor No head-to head evidence exists. Findings from placebo-controlled trials were insufficient to draw conclusions about comparative efficacy. Comparative effectiveness No evidence Major depressive disorder in children Comparative efficacy Poor No head-to head evidence exists. Findings from placebo-controlled trials were insufficient to draw conclusions about comparative efficacy. Comparative effectiveness No evidence Generalized anxiety disorder Comparative efficacy Fair to poor Available head-to head evidence is limited to comparisons of fluoxetine with sertraline and paroxetine with escitalopram or venlafaxine. Except for one study favoring escitalopram over paroxetine, no major differences in efficacy could be detected. Comparative effectiveness No evidence Second-generation antidepressants 113 of 190 Final Update 5 Report Drug Effectiveness Review Project Key Question, Disorder, and Strength of Outcome of Interest Evidence Findings Obsesssive compulsive disorder Comparative efficacy Fair to poor Available head-to head evidence is limited to comparisons of paroxetine with escitalopram, sertraline, and venlafaxine and venlafaxine with duloxetine and escitalopram. Overall, no major differences in efficacy could be detected. Comparative effectiveness No evidence Panic disorder Comparative efficacy Fair to poor Available head-to head evidence is limited to comparisons of sertraline with citalopram, nefazodone, and venlafaxine. Overall, no major differences in efficacy between citalopram and escitalopram could be detected. The evidence on the comparative efficacy of paroxetine and venlafaxine ER is inconclusive. Comparative effectiveness No evidence Post-traumatic stress disorder Comparative efficacy Fair to poor Available head-to head evidence is limited to comparisons of sertraline with citalopram, nefazodone, and venlafaxine. Overall, no major differences in efficacy could be detected. Comparative effectiveness No evidence Social anxiety disorder Comparative efficacy Fair to poor Available head-to head evidence is limited to comparisons of paroxetine with with escitalopram and venlafaxine ER. Overall, no major differences in efficacy could be detected. Comparative effectiveness No evidence Premenstrual dysphoric and late luteal phase dysphoric disorder Comparative efficacy Poor No head-to head evidence exists. Findings from placebo-controlled trials were insufficient to draw conclusions about comparative efficacy. Comparative effectiveness No evidence Key Question 2. Comparative harms of second-generation antidepressants General tolerability Adverse events profiles Fair Adverse events profiles are similar among second-generation antidepressants. Differences in the incidence of specific adverse events exist. Diarrhea Fair Evidence from multiple fair-quality studies indicates that sertraline has a higher incidence of diarrhea than bupropion, citalopram, fluoxetine, fluvoxamine, mirtazapine, nefazodone, paroxetine, and venlafaxine. Discontinuation rates Good Meta-analyses of efficacy trials indicate that overall discontinuation rates are similar.
The secondary outcome of percentage of patients who reported substantial or total symptom control did not differ significantly between the 2 drugs buy 2.5mg plendil free shipping prehypertension heart attack. The only head-to-head study investigating budesonide and mometasone for perennial rhinitis found the 2 drugs comparable for nasal symptom scores and overall symptom control 5 mg plendil with amex atrial flutter treatment. One fair-quality European RCT compared budesonide 256 mcg or 128 mcg to mometasone 200 58 mcg or placebo in 438 adults with confirmed perennial allergic rhinitis. The primary efficacy outcome, nasal symptom score (morning and evening combined), was not significantly different in the 2 medications. Furthermore, there was no statistically significant difference for the secondary outcomes: percentage of patients experiencing no symptom control, consumption of rescue medication, and onset of action. We have identified unpublished quality of life data from this study in the dossier supplied by the manufacturer of budesonide that found no significant differences between treatments except that budesonide is superior to placebo for general health and vitality. Flunisolide: New compared with old formulations The randomized double-blind parallel-group study compared 2 different formulations of flunisolide aqueous in 215 patients with confirmed perennial allergic rhinitis and found similar 59 efficacy in both treatments. Dosages were equivalent in both the old and new formulations, which reduced propylene glycol from 20% to 5%, increased polyethylene glycol from 15% to 20%, and added 2. There were no significant differences in mean reduction of total symptom and individual symptom scores between formulations. Further, patients rated acceptability of nasal burning/stinging on a 100- point visual analog scale. The original formulation had a mean score of 52 while the new formulation was rated as 87 (P<0. Outcomes in head-to-head trials of perennial allergic rhinitis patients Interventions Study (Total Daily Dose) Sample size Duration Outcome Results Reduction in mean symptom (A) -1. The only head-to-head evidence identified for triamcinolone (220 mcg) comes from an open-label randomized parallel group 3-week trial of 175 perennial allergic rhinitis patients in which there were no differences in efficacy or safety 70 endpoints when compared to fluticasone 200 mcg once daily. Indirect comparisons Placebo-controlled trials of triamcinolone were evaluated due to the dearth of head-to- head evidence available for this nasal corticosteroid. There were 4 large (N=178 to 305) fair NCS Page 28 of 71 Final Report Update 1 Drug Effectiveness Review Project quality placebo-controlled trials that assessed triamcinolone in patients with perennial allergic 71-75 rhinitis and 1 very small study of cat allergic patients (N=12). All of the larger studies reported significantly lower nasal symptoms for the active drug in treatment of perennial rhinitis. Another study of 296 patients with mixed allergic rhinitis reported -4. The 12-week placebo-controlled trial of 205 perennial rhinitis subjects taking triamcinolone aerosol 200 mcg reported change from baseline nasal index (maximum 9 points) - 74 3. A 4-week placebo-controlled trial of triamcinolone aqueous 220 mcg in 178 patients with perennial allergic rhinitis showed a significant overall reduction in nasal index (sum of 3 individual symptom scores, 4-point scale, 0=none and 3=severe) for triamcinolone compared with placebo, -2. The 1-week crossover trial of triamcinolone 220 mcg followed by a 1-hour cat allergen challenge resulted in mean nasal symptoms (4-point scale, 0=none and 73 3=severe) of 0. The effect of ciclesonide use in perennial allergic rhinitis patients was evaluated in 2 76, 77 placebo-controlled trials (see Evidence Tables 5a and 6a. Other patient demographic characteristics were similar. Only 1 of the trials was designed 76 to evaluate efficacy. In that trial, patient-rated nasal symptoms (TNSS) and quality of life (RQLQ) were both significantly improved after 6 weeks of use in the ciclesonide group compared to the placebo group. There was a slight between-group difference in physician-rated symptoms favoring ciclesonide, although this difference did not reach statistical significance. In the longer trial (52 weeks) designed to evaluate safety outcomes rTNSS scores were significantly improved from baseline compared to placebo. There was also a statistically significant difference in RQLQ scores, favoring ciclesonide, at the study¬ís endpoint. This difference was only clinically significant in the subset of patients who were more impaired at baseline (RQLQ scores 77 ¬ē3. No published effectiveness or efficacy trials of fluticasone furoate were identified. The only evidence on the efficacy of fluticasone furoate in perennial allergic rhinitis patients comes from the dossier provided by the drug¬ís manufacturer, which includes reference to 2 unpublished studies (duration of 4 and 6 weeks) evaluating symptom relief and quality of life outcomes. Compared to placebo, those patients receiving fluticasone furoate had a significant improvement in reflective TNSS in both studies. Significant improvement in ocular symptoms was not 78 observed in the 4-week study although a statistically significant improvement was observed in 79 the 6-week study.