
ECOSHELTA has long been part of the sustainable building revolution and makes high quality architect designed, environmentally minimal impact, prefabricated, modular buildings, using latest technologies. Our state of the art building system has been used for cabins, houses, studios, eco-tourism accommodation and villages. We make beautiful spaces, the applications are endless, the potential exciting.
By S. Quadir. Florida College.
Cardiac arrhythmias (Anson 2005) and sexual dysfunction have also been attributed to PIs (Schrooten 2001) tadapox 80 mg with amex impotence in men, although the data does not remain unchallenged (Lallemand 2002) tadapox 80mg overnight delivery erectile dysfunction caused by hemorrhoids. All PIs are inhibitors of the CYP3A4 system and interact with many other drugs (see chapter on Drug Interactions). Ritonavir is the strongest inhibitor, saquinavir proba- bly the weakest. There is a high degree of cross-resistance between protease inhibitors, which was described even before PIs were put on the market (Condra 1995). With darunavir and tipranavir two second-generation PIs are available that are effective even in the presence of several resistance mutations (see below). All PIs must be boostered by the so called pharmacoenhancers, in order to achieve sufficient plasma levels. Ritonavir is a potent inhibitor of the isoenzyme 3A4, a subunit of the cytochrome P450 hepatic enzyme system. Inhibition of these gastrointestinal and hepatic enzymes allows the most important pharmacokinetic parameters of almost all PIs to be significantly increased or “boosted” (Kempf 1997): maximum concentration (Cmax), trough levels (Ctrough or Cmin) and half-life. The interaction between riton- avir and the other PIs simplifies daily regimens by reducing the frequency and number of pills to be taken every day, in many cases independent of food intake. In 2014, cobicistat (Tybost) was approved as a booster for atazanavir and darunavir. Initially, cobicistat was developed for the integrase inhibitor elvitegravir in the fixed- dose combination Stribild that came to market in 2013. PK studies, however, had shown that with cobicistat comparable levels of atazanavir and darunavir can be achieved (Elion 2011, Kakuda 2014). In a double-blind, randomized study on 692 ART-naive patients treated with TDF+FTC+atazanavir, efficacy and tolerability of cobicistat and ritonavir were comparable (Gallant 2013). Based on these data, cobici- stat is now available as pharmacoenhancer for atazanavir and darunavir. More recently, the FDA and EMA have granted marketing approval to two fixed-dose com- binations. Evotaz is a combination of atazanavir and cobicistat, Prezcobix or Rezolsta contains cobicistat and darunavir. Cobicistat seems to be well-tolerated, although a slight increase of creatinine was noted. This may only be explained by a lessened tubular creatinine secretion and may not indicate an impairment of renal function (German 2013). Boosting with ritonavir or cobicistat is usually indicated by addition of an “/r” or a “/c” after the drug name (see Table 2. Resistance is only rarely observed on boosted PIs, at least in ART–naïve patients, as the genetic barrier is high. This has been shown not only for lopinavir/r (Hammer 2006), but also for fosamprenavir/r (Eron 2006), atazanavir/r (Mallan 2008), saquinavir/r (Ananworanich 2006) and darunavir/r (Ortiz 2008). Many experts therefore recommend that in highly viremic patients, prefer- ably PI/r-based regimens should be used. However, at least one large randomized study evaluating TDM- guided dose escalation of boosted PIs in almost 200 patients with extensive resist- ance mutations failed to show a significant benefit with this strategy (Albrecht 2011). There is a high degree of variability in plasma levels among individuals. As well as trough levels, peak levels are also elevated, which may lead to more side effects. If in doubt (reduced efficacy, more side effects), plasma levels should be measured in cases of boosting, especially in patients with severe hepatic disease, because the extent of interaction cannot be predetermined for individual cases. Individual agents: Special features and problems Amprenavir (APV, Agenerase) was the fifth PI to enter the market in 2000. It was replaced by fosamprenavir in 2004 (Telzir or Lexiva, see below) and subsequently withdrawn from market. Atazanavir (ATV, Reyataz, also in Evotaz) was licensed in 2004 as the first PI on the market for once daily administration. In treatment-naïve patients, atazanavir was compared to many other agents. Both boosted and unboosted atazanavir proved as effective as efavirenz (Squires 2004, Daar 2011) or nevirapine (Soriano 2011). The CASTLE study proved that virological efficacy of atazanavir/r was at least as good or even better with more favorable lipid profiles and better gastrointestinal tolerability than lopinavir/r (Molina 2008+2010).
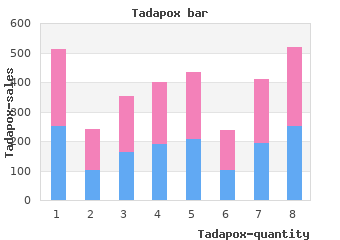
The first real step forward using ASCT in the of using a preparative transplantation regimen to destroy the setting of HIV infection came in the post-cART era in patients with lymphohematopoietic reservoir of HIV cheap tadapox 80mg on-line erectile dysfunction pills don't work. Currently buy generic tadapox 80mg online fast facts erectile dysfunction, the predominant role of HSCT treatment for relapse. It has been in this setting had viral loads 10 000 gc/mL by RT-PCR. With the exception of that parallel questions of control of malignancy and HIV have been one patient who had delayed engraftment until day 23, engraftment posed, seeking to accomplish the dual purpose of cure of the malig- of HSCs was similar to that seen in HIV-negative patients. At the nancy and control, if not cure, of the HIV infection. Although an Autologous transplantation for AIDS-related attempt was made to continue cART therapy throughout the lymphoma transplantation period, only half of the patients were able to tolerate Hematopoietic cell therapy in HIV/AIDS has been most applicable the medications due to nausea and mucositis; however, control of in the setting of AIDS-related lymphoma. Since the advent of virus after transplantation was readily achieved. This experience combination antiretroviral therapy (cART), the survival of patients was evaluated as a case-control analysis of patients with HIV- with HIV infection has improved dramatically, but as life- threatening opportunistic infections have become less common, related lymphoma compared with HIV-negative patients with the same lymphoma histologies. These results confirmed melanoma, which have increased as the HIV-infected population has another retrospective case control study comparing 53 HIV-infected aged. AIDS-defining cancers such as B-cell lymphoma are of signifi- lymphoma patients with negative controls adjusted for histology, cant concern3 and are predominantly of the B-cell subtypes having International Prognostic Index score, and disease status, demonstrat- ing overall survivals of 61% versus 70%, respectively. In addition, there has been a rise in HIV-related Hodgkin retrospective multicenter experience with 68 high risk patients with lymphoma, presumably reflecting immune stimulation driven by the HIV and lymphoma in first complete remission at 20 institutions higher CD4 counts. To address whether the Hematology 2013 389 Genetic modification of HSPC for HIV/AIDS The goal of an HSCT-based strategy for HIV/AIDS is to generate a new immune system to control HIV infection while at the same time destroying the endogenous reservoir, thereby curing the infection. Success would be largely predicated upon the creation of a durable (HIV-resistant) immune system through transplantation of innately resistant or genetically altered hematopoietic stem/progenitor cells (HSPCs); xenogeneic, allogeneic, and autologous stem cell sources have all been tested. The feasibility of transferring HIV resistance via HSPCs was demonstrated when an AIDS patient in Berlin with acute myeloid leukemia received a transplantation with HLA- matched, unrelated donor HSPCs containing a homozygous 32-bp deletion in the chemokine receptor 5 gene (CCR5 32/ 32). The recipient attained complete hematopoietic reconstitution with the donor graft, suspended cART early after transplantation, and has remained with undetectable HIV RNA in the blood and HIV DNA in the tissues using single-copy– sensitive PCR methods for at least 4 years after transplantation. Based on this case, genetic modification and engraftment of HSCs to confer HIV resistance might be a promising alternative to homozygous natural deletions in potential donors, addressing the larger question of cure of HIV in nonmalignancy HIV patients. Retroviral or lentiviral transfer of HIV resistance genes into HSCs would presumably generate progeny that are resistant to reinfection by any endogenous virus and, in the absence of a suitable reservoir, the original virus would be eliminated. Probability of disease-free survival (A) and overall 7 A phase 2 trial of gene-modified autologous cell therapy with a trial survival (B) by HIV-1 status. Recent studies show that Specimens before and after ASCT were studied with single-copy– patients undergoing high-dose chemotherapy and ASCT infused sensitive assays for HIV RNA and DNA as a surrogate measure of with a combination of gene-modified and unmodified stem cells the HIV reservoir. Despite the absence of detectable HIV RNA in could be successfully engrafted without affecting normal hematopoi- the plasma using conventional methods, 9 of the 10 patients were esis. The transplantation recipients volves autologous transplantation of CD34 cells transduced with a were likely “reinfected” with endogenous virus under cover of short hairpin RNA against CCR5; this strategy has shown success- cART, and the conclusion was that the myeloablative chemotherapy ful low-toxicity long-term downregulation of CCR5 in macaques. This finding has mC46 membrane-bound viral fusion inhibitor and a chemotherapy- prompted efforts to modify the infused T cells so that they are resistance marker has demonstrated stable protection from viral resistant to HIV. Outcome of ASCT in high-risk AIDS lymphoma for recurrent lymphoma were treated with HSPCs that had been Conditioning Time to modified by a lentivirus containing 3 different RNA-based antiretro- Study N regimens PFS follow-up viral genes without serious adverse events attributable to the 11 research HSPC product (Figure 2). This study Spitzer et al13 20 BU/CY 50% 23 wk demonstrated the safety and feasibility of the approach after Re et al9 27 BEAM 76% 24 mo myeloablative conditioning, but also showed the limitation in the Balsalobre et al10 68 Not reported 56% 32 mo ability of the autologous approach to engraft adequate numbers of Diez-Martin et al8 53 BEAM 61% 30 mo gene-modified cells. Given that clinical trials on the correction of BEAM indicates carmustine (bis-chloroethylnitrosourea-BCNU), etoposide, cytara- human genetic diseases using gene-modified HSPCs have shown bine (arabinofuranosyl cytidine), melphalan; CY, cyclophosphamide; TBI, total body success using busulfan-based regimens, these approaches are now irradiation;VP16,etoposide;BU,busulfan;andPFS,progression-freesurvival. Kaplan-Meier estimation of the survival of HIV-1–infected Figure 2. Gene marking in the peripheral blood after ASCT for patients after allogeneic HSCT during the period 1983-2010. Adding to this suggestive but finger–based strategies have the advantage of a transient cell anecdotal data is a series of patients demonstrating that all treatment ex vivo that produces a permanent genetic mutation; HIV-infected recipients of reduced intensity transplantations on preclinical development of this approach suggests that it should be cART survived, in remission and off immunosuppression at 1 year feasible in human clinical trials. Although rejection and demonstrated the feasibility of maintaining HIV- HIV DNA was readily detected in peripheral blood mononuclear infected patients on chronic immunosuppression without worsening cells before and 2 to 3 months after transplantation, HIV DNA and the underlying HIV infections as long as cART therapy is contin- RNA were undetectable in the peripheral blood mononuclear cells, ued. The “Berlin patient” index case, showing that transplantation of CD4, and plasma at 21 and 42 months after transplantation.
Phase 2 study of the safety and efficacy of vicriviroc purchase tadapox 80 mg without a prescription impotence with prostate cancer, a CCR5 inhibitor buy tadapox 80mg online erectile dysfunction surgical treatment options, in HIV- 1-Infected, treatment-experienced patients: ACTG 5211. Effect of the intensification with a CCR5 antagonist on the decay of the HIV-1 Latent reservoir and residual viremia. Efficacy and safety of maraviroc plus optimized background therapy in treatment-experienced patients infected with CCR5-tropic HIV-1: 48-week combined analysis of the MOTIVATE Studies. Two-year safety and virologic efficacy of maraviroc in treatment-experi- enced patients with CCR5-tropic HIV-1 infection: 96-week combined analysis of MOTIVATE 1 and 2. Modulation of HIV-1 co-receptor tropism and susceptibility to co-receptor inhibitors by regions outside of the V3 Loop: Effect of gp41 amino acid substitutions. The immunologic effects of maraviroc intensification in treated HIV- infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplan- tation. Predicting HIV-1 coreceptor usage with sequence analysis. CCR5-tropic resistance to maraviroc is uncommon even among patients on functional maraviroc monotherapy or with ongoing low-level replication. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tick- borne encephalitis. Maraviroc and CD4+ cell count recovery in patients with virologic suppres- sion and blunted CD4+ cell response. Changes in V3 loop sequence associated with failure of maraviroc treatment in patients enrolled in the MOTIVATE 1 and 2 Trials. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multi- ply-exposed individuals to HIV-1 infection. Current V3 genotyping algorithms are inadequate for predicting X4 co-receptor usage in clinical isolates. Maraviroc can improve lipid profiles in dyslipidemic patients with HIV: results from the MERIT trial. Epidemiology and predictive factors for chemokine receptor use in HIV- 1 infection. Assessment of immunotoxic potential of maraviroc in cynomolgus monkeys. Pichenot M, Deuffic-Burban S, Cuzin L, Yazdanpanah Y. Efficacy of new antiretroviral drugs in treatment-expe- rienced HIV-infected patients: a systematic review and meta-analysis of recent randomized controlled trials. Design and validation of new genotypic tools for easy and reliable estimation of HIV tropism before using CCR5 antagonists. Negative association between the chemokine receptor CCR5-Delta32 polymorphism and rheumatoid arthritis: a meta-analysis. Chemokine and chemokine receptor gene variants and risk of non-Hodgkin’s lymphoma in human immunodeficiency virus-1-infected individuals. A note of caution on yellow fever vaccination during maraviroc treatment: a hypothesis on a potential dangerous interaction. A double-blind, placebo-controlled trial of maraviroc in treatment-expe- rienced patients infected with non-R5 HIV-1. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine- mediated suppression. Efficacy and safety of maraviroc versus efavirenz, both with zidovu- dine/lamivudine: 96-week results from the MERIT study. Factors associated with proviral DNA HIV-1 tropism in antiretroviral therapy-treated patients with fully suppressed plasma HIV viral load: implications for the clinical use of CCR5 antagonists. Maraviroc (MVC) once daily with darunavir/ritonavir (DRV/r) in a 2-drug regimen compared to emtricitabine/tenofovir (TDF/FTC) with DRV/r: 48-week results from MODERN (Study A4001095). Impact of adding maraviroc to antiretroviral regimens in patients with full viral suppression but impaired CD4 recovery. Response to vicriviroc (VCV) in HIV-infected treatment-experienced subjects using an enhanced Trofile HIV co-receptor tropism assay: reanalysis of ACTG 5211 results.
Papakostas GI purchase tadapox 80mg amex erectile dysfunction icd 9 2014, Montgomery SA tadapox 80mg on-line zinc causes erectile dysfunction, Thase ME, Katz JR, Krishen A, Tucker VL. Comparing the rapidity of response during treatment of major depressive disorder with bupropion and the SSRIs: a pooled survival analysis of 7 double-blind, randomized clinical trials. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. Venlafaxine XR demonstrates higher rates of sustained remission compared to fluoxetine, paroxetine or placebo. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Second-generation antidepressants 140 of 190 Final Update 5 Report Drug Effectiveness Review Project 369. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. Thase ME, Clayton AH, Haight BR, Thompson AH, Modell JG, Johnston JA. A double- blind comparison between bupropion XL and venlafaxine XR: sexual functioning, antidepressant efficacy, and tolerability. A randomized, double-blind, 24-week study comparing the efficacy and tolerability of mirtazapine and paroxetine in depressed patients in primary care. Serotonin selective reuptake inhibitors in child and adolescent psychopharmacology: a review of published experience. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Carmody T, Mayes TL. Fluoxetine in child and adolescent depression: acute and maintenance treatment. Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? A double-blind comparison of escitalopram and paroxetine in the long-term treatment of generalized anxiety disorder. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. Efficacy, safety, and tolerability of venlafaxine XR in generalized anxiety disorder. Analysis of the rate of improvement of specific psychic and somatic symptoms of general anxiety disorder during long-term treatment with venlafaxine ER. Estimation of symptom-free days in generalized anxiety disorder. Clomipramine, fluoxetine, and behavior therapy in the treatment of obsessive-compulsive disorder: a meta-analysis. Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. Kobak KA, Greist JH, Jefferson JW, Katzelnick DJ, Henk HJ. Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Nair NP, Bakish D, Saxena B, Amin M, Schwartz G, West TE. Comparison of fluvoxamine, imipramine, and placebo in the treatment of outpatients with panic disorder. Second-generation antidepressants 141 of 190 Final Update 5 Report Drug Effectiveness Review Project 384. Efficacy and tolerability of mirtazapine and sertraline in Korean veterans with posttraumatic stress disorder: a randomized open label trial.
Study of binding and neutralising antibodies to interferon-beta in two groups of relapsing-remitting multiple sclerosis patients 80mg tadapox overnight delivery erectile dysfunction cpt code. Neutralizing and binding anti-interferon-beta (IFN-beta) antibodies order tadapox 80 mg mastercard back pain causes erectile dysfunction. A comparison between IFN-beta-1a and IFN-beta-1b treatment in multiple sclerosis. Koch-Henriksen N, Sorensen PS, Bendtzen K, Flachs EM. The clinical effect of neutralizing antibodies against interferon-beta is independent of the type of interferon- beta used for patients with relapsing-remitting multiple sclerosis. Predictive markers for response to interferon therapy in patients with multiple sclerosis. Neutralizing antibodies explain the poor clinical response to interferon beta in a small proportion of patients with multiple sclerosis: a retrospective study. Randomized study of once-weekly interferon beta-1la therapy in relapsing multiple sclerosis: three-year data from the OWIMS study. Eight-year immunogenicity and safety of interferon beta-1a-Avonex treatment in patients with multiple sclerosis. Martinelli V, Gironi M, Rodegher M, Martino G, Comi G. Occurrence of thyroid autoimmunity in relapsing remitting multiple sclerosis patients undergoing interferon- beta treatment. Effect of 1-year treatment with interferon- beta1b on thyroid function and autoimmunity in patients with multiple sclerosis. Longitudinal analyses of the effects of neutralizing antibodies on interferon beta-1b in relapsing-remitting multiple sclerosis. A multicenter, open-label, phase II study of the immunogenicity and safety of a new prefilled syringe (liquid) formulation of Avonex in patients with multiple sclerosis. Neutralizing antibodies in multiple sclerosis patients treated with 375 microg interferon-beta-1b. Disease-modifying drugs for multiple sclerosis Page 91 of 120 Final Report Update 1 Drug Effectiveness Review Project 116. Are ex vivo neutralising antibodies against IFN-beta always detrimental to therapeutic efficacy in multiple sclerosis? The incidence and significance of anti- natalizumab antibodies: results from AFFIRM and SENTINEL. Clerico M, Faggiano F, Palace J, Rice G, Tintore M, Durelli L. Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. The effects of intramuscular interferon beta-1a in patients at high risk for development of multiple sclerosis: a post hoc analysis of data from CHAMPS. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. The controlled high risk Avonex multiple sclerosis trial (CHAMPS Study). Journal of neuro-ophthalmology : the official journal of the North American Neuro- Ophthalmology Society. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double- blind, placebo-controlled trial. Interferon beta-1a for optic neuritis patients at high risk for multiple sclerosis. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study.