
ECOSHELTA has long been part of the sustainable building revolution and makes high quality architect designed, environmentally minimal impact, prefabricated, modular buildings, using latest technologies. Our state of the art building system has been used for cabins, houses, studios, eco-tourism accommodation and villages. We make beautiful spaces, the applications are endless, the potential exciting.
2018, Antioch University Seattle, Mamuk's review: "Exelon generic (Rivastigimine) 6 mg, 4.5 mg, 3 mg, 1.5 mg. Effective online Exelon no RX.".
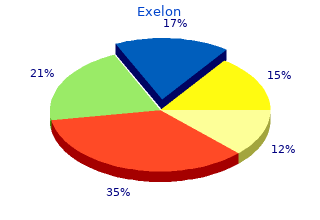
Readers should refer to the Health Canada product monograph of individual drug products for dosing information for Canada order exelon 3 mg line symptoms 8 dpo bfp. Targeted immune modulators work by selectively blocking mechanisms involved in the inflammatory and immune response discount 1.5mg exelon with visa medicine 832. Tumor necrosis factor inhibitors block specific proinflammatory mediators known as cytokines. Adalimumab, certolizumab pegol, golimumab, Targeted immune modulators 11 of 195 Final Update 3 Report Drug Effectiveness Review Project and infliximab all bind to both the circulating and transmembrane forms of tumor necrosis factor alpha, inhibiting its biological activity. Adalimumab is a fully human monoclonal antibody that blocks tumor necrosis factor alpha’s interaction with both the p55 and p75 cell surface tumor necrosis factor receptor. Certolizumab pegol is a recombinant, humanized antibody FAB fragment with specificity for human tumor necrosis factor alpha. Golimumab is a human monoclonal antibody that binds to tumor necrosis factor alpha. Infliximab is a chimeric (mouse/human) antitumor necrosis factor alpha antibody. Etanercept is a soluble dimeric form of the p75 tumor necrosis factor alpha receptor linked to the Fc portion of human immunoglobulin G1. It exerts its action by binding circulating tumor necrosis factor alpha and lymphotoxin-α and preventing it from interacting with a cell surface receptor. Interleukin-1, another naturally occurring cytokine, has both immune and pro inflammatory actions. Anakinra is a human recombinant protein and the therapeutic version of a naturally occurring cytokine that competitively blocks the interleukin-1 receptor, thus blocking various inflammatory and immunological responses. The immunosuppressant agents abatacept and alefacept exert their immune regulation by interfering with T lymphocyte activation and efalizumab blocks lymphocyte activation and migration. Abatacept is a soluble fusion protein that consists of the extracellular domain of human cytotoxic T lymphocyte-associated antigen (CTLA-4) and the modified Fc portion of immunoglobulin G1. Alefacept is a dimeric fusion protein that consists of the extracellular CD2- binding portion of the human leukocyte function antigen (LFA-3) and the Fc portion of human immunoglobulin G1. Efalizumab is a recombinant humanized immunoglobulin G1 monoclonal antibody that binds to human CD11a and inhibits the binding to intercellular adhesion molecule- 1 (ICAM-1). Progressive multifocal leukoencephalopathy is a rapidly progressive, viral infection of the central nervous system that leads to death or severe disability. Because it is unclear whether efalizumab will be reintroduced to the United States market, we will not discuss the use of efalizumab in this report any further. Natalizumab is a recombinant immunoglobulin G4 antibody that binds to the alpha 4 subunit of alpha 4β1 and alpha4β7 integrins expressed on the surface of all leukocytes except neutrophils. Because of an increased risk of progressive multifocal leukoencephalopathy, natalizumab is only available through a specialized TM TM restricted distribution program called TOUCH Prescribing Program. Under the TOUCH Prescribing Program only prescribers, infusion centers, and pharmacies registered with the program are able to prescribe, distribute, and infuse the product. Rituximab, a chimeric murine/human monoclonal antibody, works by binding to the CD20 antigen found on the surface of B lymphocytes. B-cells are believed to play a role in autoimmune and inflammatory processes, such as those involved in rheumatoid arthritis. Tocilizumab is a recombinant humanized monoclonal antibody against the interleukin-6 receptor. Interleukin-6 is a pro inflammatory cytokine produced by a variety of cell types including T- and B-cells, lymphocytes, monocytes, and fibroblasts and has been shown to play a role in immune response, such as those involved in autoimmune diseases. Finally, ustekinumab is a human monoclonal antibody that binds to the p40 protein subunit used by both the interleukin-12 and interleukin-23 cytokines. Interleukin-12 and Targeted immune modulators 12 of 195 Final Update 3 Report Drug Effectiveness Review Project interleukin-23 are naturally occurring cytokines that are involved in inflammatory and immune responses. In this report, we review the comparative effectiveness, safety, and tolerability of targeted immune modulators. Our review covers the use of these drugs in adult patients with rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and pediatric patients with juvenile idiopathic arthritis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
There is more experience with this combination than with any other buy exelon 3mg lowest price symptoms 4dp5dt fet. The resistance profile is favorable: the M184V mutation that frequently develops during 3TC treatment increases sensitivity to AZT exelon 6mg mastercard treatment renal cell carcinoma. Although the licensing study for Combivir showed no difference in toxicity (Eron 2000), in our experience the 300 mg AZT dose in Combivir is too high for some patients and can lead to anemia. In such cases, it is worth trying AZT+3TC as individual components, so that the dose of AZT can be reduced to 250 mg BID. AZT+3TC has comparable efficacy to d4T+3TC or to AZT+FTC (Benson 2004). The ACTG 384 Study showed superiority of AZT+3TC over d4T+ddI (Robbins 2003, Shafer 2003). However, this notion did change over time: while early results suggested a lower rate of lipoatrophy (Molina 1999), the development of lipoatrophy with AZT+3TC occurred only slightly later than with d4T+ddI. AZT+3TC was shown to be less effective and less well-tolerated than TDF+FTC in the GS-934 study (Gallant 2006, Pozniak 2006). Another large ACTG study also showed that it was less well- 78 ART tolerated (Campbell 2011). Compared to ABC+3TC, immune reconstitution may be less impressive (DeJesus 2004). Facing these potential disadvantages and the fact that once daily dosing is not possible, most guidelines no longer recommend AZT+3TC as a preferred backbone in treatment-naïve patients. Some studies suggest a comparable efficacy (and better tolerability) versus AZT+3TC (Berenguer 2008). However, keeping in mind the long-term toxicity of ddI, we would only recommend ddI+3TC when there are significant reasons to not use TDF+FTC or ABC+3TC. Poor and not-recommended backbones It should be noted that the majority of the clinical trials cited above were conducted in treatment-naïve patients. In pretreated patients, other backbones may be neces- sary due to resistance or lack of tolerability. But the following backbones should be avoided whenever possible: Guidelines explicitly recommend avoiding the previously very popular combination of d4T+ddI and of d4T+3TC. Mitochondrial toxicity is high, the use of d4T can no longer be justified. Increased gastrointestinal side effects and the necessity of taking ddI on an empty stomach (AZT is better tolerated taken with a meal) speak against the combination AZT+ddI. Due to their divergent resistance pathways AZT+TDF is not recommended for primary therapy and should be restricted to treatment-experienced patients only. The combination TDF+ddI is relatively toxic and over the years many studies have shown less virologic and immunologic efficacy (see section on Inappropriate Initial Therapies). TDF+ABC are problematic due to rapid development of resistance. AZT+d4T and FTC+3TC are antagonistic (competitive, as noted above) and should not be employed. Alternating backbones with regular changes from one backbone to another can not currently be recommended, although initial studies indicate that this strategy is at least not harmful (Molina 1999, Martinez-Picado 2003). References Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV- infected adults with raised cholesterol: effect on lipid profiles. A randomized study of emtricitabine and lamivudine in stably sup- pressed patients with HIV. Didanosine, lamivudine, and efavirenz versus zidovudine, lamivudine, and efavirenz for the initial treatment of HIV type 1 infection: final analysis (48 weeks) of a prospective, ran- domized, noninferiority clinical trial, GESIDA 3903. Comparative effectiveness of continuing a virologically effective first- line boosted protease inhibitor combination or of switching to a three-drug regimen containing either efavirenz, nevirapine or abacavir. Antiretroviral treatment simplification with 3 NRTIs or 2 NRTIs plus nevi- rapine in HIV-1-infected patients treated with successful first-line HAART.