
ECOSHELTA has long been part of the sustainable building revolution and makes high quality architect designed, environmentally minimal impact, prefabricated, modular buildings, using latest technologies. Our state of the art building system has been used for cabins, houses, studios, eco-tourism accommodation and villages. We make beautiful spaces, the applications are endless, the potential exciting.
2018, Wingate University, Grubuz's review: "Prevacid generic (Lansoprazole) 30 mg, 15 mg. Proven Prevacid online.".
Generation of a cloned NK cell line derived from the “null 23 generic prevacid 15 mg free shipping gastritis diet �����������. Expanded natural killer cell” fraction of human peripheral blood 15mg prevacid fast delivery diet for gastritis and diverticulitis. Good manufacturing cells: improvement of clinical responses in metastatic renal cell practice-compliant cell sorting and large-scale expansion of carcinoma patients previously treated with IL2. Ex-vivo expansion of NK cells: what is the receptors and death receptor ligands and have enhanced cyto- priority–high yield or high purity? Luhm J, Brand JM, Koritke P, Hoppner M, Kirchner H, Frohn 42. Large-scale generation of natural killer lymphocytes for tumor cell line, HFWT, can greatly stimulate proliferation of clinical application. Natural killer cell engineering for activation and expansion of natural killer cells from patients cellular therapy of cancer. Successful transfer of primary natural killer cells overcomes inhibitory signals and alloreactive haploidentical KIR ligand-mismatched natural killer induces specific killing of leukemic cells. Activating signals dominate cytotoxic human natural killer cells for cancer cell therapy. Large-scale ex vivo NK cells have increased natural cytotoxity receptors, TRAIL expansion and characterization of natural killer cells for clinical and NKG2D expression, and superior tumor cytotoxicity com- applications. Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Fresh ex vivo expanded cal NK cells in patients with cancer. Allogeneic high-risk relapsed multiple myeloma (MM) patients [abstract]. Cancer Immunol Blood (ASH Annual Meeting Abstracts). Hemolytic anemia due to adoptively transferred ex-vivo expanded autologous natural passenger lymphocyte syndrome in solid malignancy patients killer (NK) cells following treatment with bortezomib to treated with allogeneic natural killer cell products. Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri immunotherapy with purified natural killer cells after haploiden- G. Preferential proliferation of natural killer cells among tical SCT: a prospective phase II study in two centers. Bone peripheral blood mononuclear cells cocultured with B lympho- Marrow Transplant. Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Increased proliferation, lytic activity, and purity of human J Clin Oncol. Bortezomib and for adoptive immunotherapy using an automated bioreactor. Autologous antitumor killer cell tumor cytotoxicity. Nicotinamide, a form of treated tumors sensitized to NK cell apoptosis paradoxically Hematology 2013 245 acquire resistance to antigen-specific T cells. Expansion, circulating natural killer cells but does not mediate tumor purification, and functional assessment of human peripheral regression. IL-18-induced aAPC Activated NK-DLI following allogeneic PBSCT in CD83 CCR7 NK helper cells. Ex-vivo expanded human NK lymph node homing of ex vivo-expanded human natural killer cells express activating receptors that mediate cytotoxicity of cells via trogocytosis of the chemokine receptor CCR7. Mamcarz E, Berg M, Peled T, Frei G, Reger R, Childs R. Nicotinamide (NAM) increases surface expression of CD62L 56.
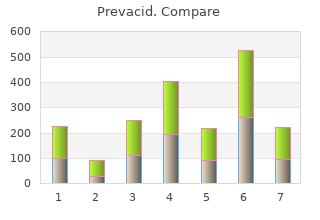
Illingworth generic prevacid 30 mg gastritis virus, 2001 Fair-LDL-lowering discount prevacid 30mg otc gastritis diet and exercise, Fair-good-safety Insull W, 2007 (SOLAR) Fair Insull, 2001 Poor-equivalent doses not compared. Statins Page 301 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Study or Author Randomization Allocation Eligibility criteria Outcome assessors Care provider Year adequate? Jacotot, 1995 Yes Not reported Yes, for height, weight, BMI Yes Yes Yes Jones,1998 Yes Not reported Yes-not much detail. Yes No No LDL-c slightly lower for 3 of 4 atorva groups. Jukema, 2005 Method not reported Not reported Yes Yes No-open label No- open label Kai T, 2008 Not randomized Open-Label Before and After, so Yes Yes No-open label No-open label Karalis, 2002 Method not reported Not reported Some differences- more men Yes Yes Not reported in atorva 10mg than simva 20mg, and BP higher in simva vs atorva group. Lloret R, 2006 Method NR NA Yes Yes No - open label No - open label (STARSHIP trial) Statins Page 302 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Patient Different or overall high Study or Author unaware of Intention-to-treat Maintained Reported attrition, crossovers, loss to follow- Year treatment? Jacotot, 1995 Yes Yes and on treatment Yes Attrition-yes, crossovers-no, adherence-no, No analysis too. No Kai T, 2008 No-open label Yes Yes No Not reported Karalis, 2002 No No Not enough detail No Not reported provided Lloret R, 2006 No - open label Yes Yes Attrition-56 (8. Internal validity of controlled clinical trials Study or Author Score Year (good/ fair/ poor) Jacotot, 1995 Fair-LDL lowering. Fair-safety although no doses provided at which adverse effects occurred. Small sample size in certain groups and LDL-c was lower for 3 out of 4 atorva groups. Jukema, 2005 Fair Kai T, 2008 Fair-poor Small sample size. The patients were compared against their own baseline scores while on simvastatin, no real comparison group. Karalis, 2002 Poor- differences at baseline, randomization and allocation methods not reported, not ITT, withdrawals not clear. Lloret R, 2006 Fair (STARSHIP trial) Statins Page 304 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Study or Author Randomization Allocation Eligibility criteria Outcome assessors Care provider Year adequate? Marz,1999 Yes Not reported Yes Yes Yes-serious adverse No effects Mazza F, 2008 Method NR NA Yes Yes NA - open label NA - open label Milionis H, 2006 Method NR NA Yes Yes NR NR (ATOROS study) Mulder D, 2007 Method NR NR NO BMI was sig more in Yes NR NR atorva Murakami T, 2006 NR NR Yes-minimal Yes-minimal NR NR Nash,1996 Yes Not reported No-higher rate of musculo- Yes No No skeletal conditions in lova group. Olsson, 2003 Method not reported Not reported Yes Yes Yes Yes Ose, 1995 Yes Not reported Yes Yes Yes Yes Statins Page 305 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Patient Different or overall high Study or Author unaware of Intention-to-treat Maintained Reported attrition, crossovers, loss to follow- Year treatment? Marz,1999 No Do not know Yes Attrition-reported, crossovers-no, adherence- No no, contamination-no Mazza F, 2008 NA - open label Yes Yes Attrition-no, crossovers-no, adherence-no, No contamination-no Milionis H, 2006 NA Yes Yes Attrition-yes, crossovers-no, adherence-no, No (ATOROS study) contamination-no Mulder D, 2007 NR No Yes Attrition-yes, crossovers-no, adherence-yes, 16 dropped and 44 others contamination-no excluded (total 26%) Murakami T, 2006 Yes No NR Attrition-yes, crossovers-no, adherence-yes, Not reported contamination-no Nash,1996 No Yes No-higher Attrition-yes, crossovers-no, adherence-yes, No musculoskeletal contamination-no conditions in lova. Olsson, 2003 Yes No Yes Attrition and adherence yes, others no No Ose, 1995 Yes No Yes Attrition-yes, crossovers-no, adherence-yes, No contamination-no Statins Page 306 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Study or Author Score Year (good/ fair/ poor) Marz,1999 Fair-LDL-lowering, Fair-safety although no details on dose at which adverse effects occurred. Mazza F, 2008 Fair Milionis H, 2006 Fair (ATOROS study) Mulder D, 2007 Poor- lack of ITT and high loss to follow up. Poor-safety since higher rate of musculo-skeletal conditions in lova group. Also no doses at which adverse effects in fluva group occurred. Statins Page 307 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 6. Internal validity of controlled clinical trials Study or Author Randomization Allocation Eligibility criteria Outcome assessors Care provider Year adequate? Paragh, 2004 Yes, though method Not reported Not reported Yes No - open label Not reported - open not reported label Recto, 2000 Yes Not reported Yes Yes No No Saklamaz, 2005 Method not reported Not reported Yes Yes Not reported Not reported Schaefer, 2003 Method not reported Not reported - open Yes Yes No - open label Not reported - open label label Schulte, 1996 Yes Not reported Yes Yes Yes Yes Schuster, 2004 Yes Not reported Yes Yes No - open label Not reported - open label Schwartz, 2004 Yes Not reported Yes Yes Yes Not reported Sigurdsson, 1998 Method not reported Not reported Simva group slightly older Yes Yes Not reported (61. Internal validity of controlled clinical trials Patient Different or overall high Study or Author unaware of Intention-to-treat Maintained Reported attrition, crossovers, loss to follow- Year treatment?
In addition generic prevacid 30mg without a prescription atrophic gastritis symptoms mayo, details should be reported relating to the process of decision-making buy prevacid 30 mg fast delivery gastritis diet ��������, i. Is there evidence of a substantial effort to search for all relevant research? Skeletal Muscle Relaxants Page 234 of 237 Final Report Update 2 Drug Effectiveness Review Project This is usually the case if details of electronic database searches and other identification strategies are given. Ideally, details of the search terms used, date and language restrictions should be presented. In addition, descriptions of hand-searching, attempts to identify unpublished material, and any contact with authors, industry, and research institutes should be provided. The appropriateness of the database(s) searched by the authors should also be considered, e. Is the validity of included studies adequately assessed? A systematic assessment of the quality of primary studies should include an explanation of the criteria used (e. Authors may use either a published checklist or scale, or one that they have designed specifically for their review. Again, the process relating to the assessment should be explained (i. Is sufficient detail of the individual studies presented? If a paper includes a table giving information on the design and results of the individual studies, or includes a narrative description of the studies within the text, this criterion is usually fulfilled. If relevant, the tables or text should include information on study design, sample size in each study group, patient characteristics, description of interventions, settings, outcome measures, follow-up, drop-out rate (withdrawals), effectiveness results and adverse events. The authors should attempt to synthesize the results from individual studies. In all cases, there should be a narrative summary of results, which may or may not be accompanied by a quantitative summary (meta-analysis). For reviews that use a meta-analysis, heterogeneity between studies should be assessed using statistical techniques. If heterogeneity is present, the possible reasons (including chance) should be investigated. In addition, the individual evaluations should be weighted in some way (e. Skeletal Muscle Relaxants Page 235 of 237 Final Report Update 2 Drug Effectiveness Review Project Appendix D. Quality abstraction tool for adverse events of muscle relaxants Author Study ____ Year published Citation Setting (country, single or multicenter, specialty or primary care clinic) Type of study (RCT, crossover, population-based, retrospective cohort, prospective cohort) INTERNAL VALIDITY Selection: 1: Study states "all patients" or "consecutive series" during specified time period (observational study) or describes and accounts for all patients deemed eligible (clinical trial) and has explicit inclusion and exclusion criteria applied to all eligible patients (all study types) 0: Selection not clear, biased selection, inclusion and exclusion criteria not specified, or unable to determine proportion of patients eligible for trial who withdrew or were not entered Loss to follow-up: 1: Low overall and differential loss to follow-up (<15% of study population or <25% difference between groups), able to compute adverse effects according to intention-to-treat if low loss to follow- up 0: High overall or differential loss to follow-up (>15% overall or >25% difference between groups), or unable to calculate intention- to-treat if low loss to follow-up Adverse events pre-specified and pre-defined: 1: Study reports definitions used for assessed adverse events in an explicit, reproducible fashion 0: Study does not meet above criteria Ascertainment techniques adequately described: 1: Study reports methods used to ascertain complications, including who ascertained, timing, and methods used 0: Study does not meet above criteria Non-biased and accurate ascertainment of adverse events: 1: Patients and assessors blinded to intervention and ascertainment techniques go beyond patient self-report alone 0: Study does not meet above criteria Statistical analysis of potential confounders: 1: Study examines more than 2 relevant confounders/risk factors using standard acceptable statistical techniques 0: Study does not meet above criteria Adequate duration of follow-up: Skeletal Muscle Relaxants Page 236 of 237 Final Report Update 2 Drug Effectiveness Review Project Appendix D. Quality abstraction tool for adverse events of muscle relaxants (continued) EXTERNAL VALIDITY Adequate description of study population: 1: Study reports 2 or more demographic characteristics and both basic clinical characteristics of pain syndrome and average duration of pain 0: Study does not meet above criteria Does study report numbers screened and eligible (trial) or inception cohort (observational study)? Are exclusion criteria specified and numbers excluded for each criteria reported? Are patients in the study on opioids prior to study entry? Reports are not usage guidelines, nor should they be read as an endorsement of or recommendation for any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Update 3: November 2006 Update 2: May 2004 Update 1: September 2003 Original Report: May 2002 The literature on this topic is scanned periodically Kim Peterson, MS Marian McDonagh, PharmD Sujata Thakurta, MPA: HA Tracy Dana, MLS Carol Roberts, BS Roger Chou, MD Mark Helfand, MD, MPH Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Copyright © 2010 by Oregon Health & Science University Portland, Oregon 97239. Final Report Update 4 Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. Prior versions of this report can be accessed at the DERP website. Nonsteroidal antiinflammatory drugs (NSAIDs) 2 of 72 Final Report Update 4 Drug Effectiveness Review Project STRUCTURED ABSTRACT Purpose We compared the effectiveness and harms of oral or topical nonsteroidal antiinflammatory drugs (NSAIDs) in the treatment of chronic pain from osteoarthritis, rheumatoid arthritis, soft tissue pain, back pain, and ankylosing spondylitis. Data Sources We searched Ovid MEDLINE and the Cochrane Library and the Database of Abstracts of Reviews of Effects through May 2010. For additional data we also hand searched reference lists, US Food and Drug Administration medical and statistical reviews and dossiers submitted by pharmaceutical companies. Review Methods Study selection, data abstraction, validity assessment, grading the strength of the evidence, and data synthesis were all carried out according to standard Drug Effectiveness Review Project review methods.
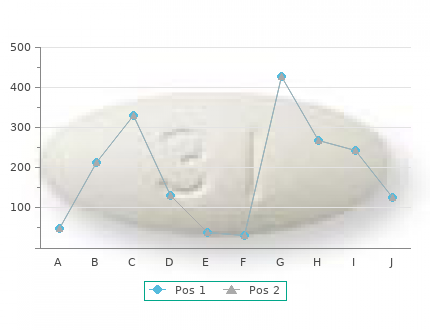
It is also used in higher Paclitaxel is metabolized in the kidney and biliary doses as palliative chemotherapy in recurrent cancer excretion is important 30mg prevacid with mastercard chronic gastritis curable. In high doses it is a component of the the most important side-effect order prevacid 15 mg with visa definition de gastritis, but the incidence is BEP regime for germ cell tumors of the ovary as well greatly reduced by adequate pre-mediation with as part of the EMA/EP regime for choriocarcinoma. Neutropenia is the main dose-limiting cursors, inter- and intra-strand alkylation and non- toxicity. The nadir occurs 8–11 days after adminis- specific cell cycle phase activity. Administration is tration and recovery occurs within 20 days. Cisplatin is cleared by the kidneys side-effects are alopecia, peripheral neurotoxicity mainly by glomerular filtration. Normal renal func- (mainly neurosensory), peri-treatment myalgia tion must be ensured before administration. The which responds to simple analgesics, asymptomatic most common toxicities are nausea, vomiting and bradycardia, mucositis and inflammation at the in- renal dysfunction. The renal toxicity can be decreased by ensuring rapid clearance of the drug by saline or Methotrexate mannitol diuresis. This has been described under This is a folate antagonist and acts by binding to the section on side-effects. Cellular protein synthesis, urinary output must be maintained during the 24h DNA and RNA production and cellular replica- following infusion, maintaining a urinary output of tion is affected. Leucovorin rescue is used 24 h after at least 100ml/h. Other toxicities are ototoxicity administration of methotrexate to reduce the toxic (irreversible) and peripheral sensory neuropathy. The drug is mostly excreted by the kidney with a small amount through Carboplatin bile. Decreased renal function can result in toxi- This is a platinum compound closely related to cis- city. It has replaced cisplatin in many of the urine alkalinized to reduce renal side-effects. Vomiting, diarrhea, stomatitis and actions and fever. Other side-effects are pulmo- interstitial pneumonitis and lung fibrosis. Doxorubicin, Adriamycin, epirubicin Cyclophosphamide These are anti-tumor antibiotics and their anti- It is an alkylating agent and is largely metabolized cancer actions are by topoisomerase II inhibition, in the liver into active compounds. It is excreted in DNA intercalation and free radical formation. Myelosuppression occurs 8–14 days after Anthracyclines are the major cornerstones of administration. Nausea and vomiting can be chemotherapy treatment in breast cancer. Doxo- delayed, occurring 6–8h after administration, so rubicin is used together with ifosfamide in one of anti-emetic prophylaxis should be given for 24h. Alopecia, skin and nail changes, increased liver It is metabolized in the liver. Most of the drug after enzymes and rarely jaundice can occur. Hemor- metabolism is excreted in the bile with smaller rhagic cystitis (adequate hydration decreases the amounts in the urine. Dose reductions are recom- incidence) and secondary leukemia are other side- mended for hyperbilirubinemia (1. Toxicities Ifosfamide include myelosuppression, nausea, vomiting, mucositis and stomatitis, alopecia, red or pink- This is an alkylating agent and is activated in the colored urine and hyperpigmentation.